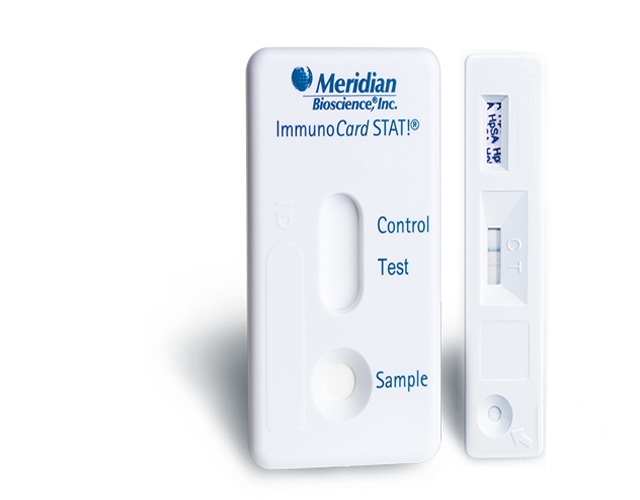
Definitive answers, confidence in results
Definitive answers, confidence in results
Related Tests
- Gastrointestinal Immunocard STAT!® HpSA® Immunocard STAT!® Rotavirus Immunocard STAT!® EHEC Immunocard STAT!® E. coli O157 Plus Immunocard STAT!® CAMPY
- Parasitology Immunocard STAT!® Crypto/Giardia Controls Immunocard STAT!® Crypto/Giardia
- Pediatric & Neonatal Immunocard STAT!® MONO
- Respiratory Immunocard STAT!® Strep A Immunocard STAT!® Flu A&B Immunocard STAT!® Flu A&B Nasopharyngeal Wash/Aspirate Accessory Kit
- Sexual Health Immunocard STAT!® hCG
Support & Documents
Downloadable PDFs
Webinars & Videos
FAQs
Helicobacter pylori (H. pylori) is a bacterium that lives on the lining of the stomach. Although we used to think that spicy food, acid, and stress were the major causes of ulcers, we now know that 9 out of 10 ulcers are caused by H.pylori. Medicines that reduce stomach acid may make you feel better, but your symptoms may come back. Here’s the good news – since most ulcers are caused by this bacterial infection, it can be cured with the right antibiotics.
There are 3 types of recommended tests:
- Stool Test: Provide a small stool specimen (an easy-to-use collection system can be provided for this purpose). The doctor will conduct the test in his office or send it to a laboratory. HpSA is the #1 stool antigen test in the USA.
- Breath Test: In this test, you have to fast then a breath sample is taken after you wait and drink a 13C-labeled urea mix. The breath sample collected is then analyzed in the office or sent out to a laboratory.
- Endoscopy: This is a test in which a small tube with a camera inside is inserted through the mouth an into the stomach to look for ulcers. During The endoscopy, small samples of the stomach lining can be obtained and tested for H. pylori.
Please refer to our H. pylori Learning Center website to view the AGA/ACG Guideline flowchart at www.hpylorilearningcenter.com/Testing
Test it. Treat it. Test it again – all with one easy stool test. The HpSA test is the only CLIA-waived FDA cleared 3-in-1 test able to diagnose H. pylori, monitor if therapy is working and test for eradication.
No, only fresh or frozen stool samples. The product is not validated for the use of samples in Cary Blair. The 1:4 dilution of Cary Blair increases the Limit of Detection and could cause false negative results.
- https://www.nphl.org/documents/ShigaToxinTestingRecommendations.pdf
- CDC Recommendations and Reports MMWR October 16, 2009 / 58(RR12);1-14
- Morbidity and Mortality Weekly Report. 2013;62(50):1029-1031
Para-Pak
The Para-Pak system provides a standardized procedure for the routine collection, transport, preservation, and culture of stool specimens for bacterial enteric pathogens. Learn More.
Pricing
To order, Contact your regional sales representative or use the following contact information:
- Email: sales@meridianbioscience.com
- Phone: +1 800 696-0739
- International Sales Extension: +1 513 271-3000