The 2025 flu season has been more intense than recent years, with elevated infection rates and notable pressure on healthcare systems1. Alongside seasonal influenza, several emerging respiratory viruses are gaining attention from global health authorities, including human metapneumovirus (HMPV), avian influenza (H5N1), and novel coronaviruses.
As respiratory threats continue to evolve, so must our strategies for detection and response. With transmission patterns influenced by factors like global travel, urban density, and climate shifts, diagnostics play a central role—enabling faster identification, targeted treatment, and more effective outbreak management through tools such as POCT, syndromic panels, and genomic surveillance.
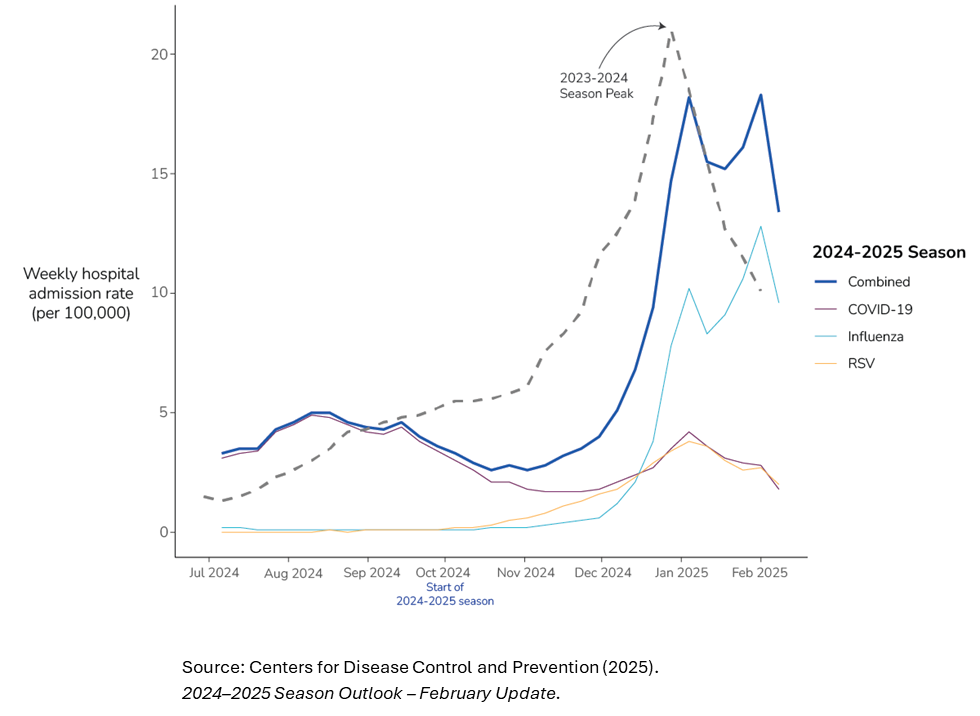
National weekly hospital admission rates (per 100,000) for COVID-19, influenza, and RSV in the 2024/2025 respiratory season
Key Trends from the 2025 Flu Season
A closer look at the 2024/2025 flu season reveals key trends in virus transmission, healthcare impact, and vaccine performance—reflected in rising test positivity rates, peak hospitalization data, and age-stratified vaccine effectiveness studies.
- High Positivity Rates:
Over 30% of flu tests were positive, indicating widespread community transmission—similar to 2009 pandemic levels2. - Hospitalization Surge:
Rates peaked at 14 per 100,000 in early February, signaling significant strain on healthcare systems³. - Variable Vaccine Effectiveness:
Adult vaccine effectiveness ranged from 36–55%, while rates in children and adolescents reached 59–78%⁴. - Geographic Differences in Virus Activity:
Flu peaked earlier in the southern U.S. and Australia, while northern regions and parts of Europe faced later but intense surges in flu and RSV. COVID-19 hospitalizations were more consistent across regions but spiked in major urban centers5. - 支原体肺炎 Activity Declines:
After gaining attention in 2024, global cases fell back to baseline by early 2025. North America saw only mild increases, while outbreaks in China and parts of EMEA subsided6. - Rise of HMPV:
Human metapneumovirus gained attention in late 2024 and early 2025, particularly in China, where it accounted for more than 6% of positive respiratory illness tests7. - Persistent Avian Influenza Activity:
Ongoing H5N1 outbreaks in poultry since 2022 continue to raise concern for zoonotic transmission8. - Surveillance of Novel Coronaviruses:
A newly identified bat coronavirus (HKU5-CoV-2) showed potential for human infection via the ACE2 receptor, prompting heightened monitoring9.
Overall, compared to recent years, the early and sustained rise in flu positivity rates, along with varied vaccine effectiveness across age groups, signals a shift in how seasonal influenza is affecting public health. For diagnostic assay developers, this highlights the need for sensitive and flexible solutions that can adapt to changing transmission patterns and the presence of multiple circulating pathogens. As a result, demand is increasing for rapid, scalable, and accessible assays—including point-of-care formats—that can accurately distinguish between flu, COVID-19, RSV, and emerging viruses like HMPV.
Preparing for the Unpredictable: The Role of Diagnostics in Emerging Respiratory Viruses
Vaccination remains the cornerstone of prevention for respiratory viruses like influenza, RSV, and COVID-19, but typically targets only known pathogens and dominant strains—providing only partial protection. As new respiratory viruses emerge, such as HMPV, avian influenza (H5N1), and novel coronaviruses, a major challenge in global health is preparing for threats that are difficult to predict. Some viruses cause mild illness, while others may evolve with the potential to trigger widespread outbreaks. However, vaccine development is not feasible or necessary for every emerging virus, making other public health measures essential10,11. As a result, early detection, surveillance, and adaptable diagnostics play a vital role in managing new and unpredictable threats.
Respiratory testing is a disease area at the center of diagnostic innovation, especially following the pandemic, which accelerated the shift toward at-home and point-of-care testing. This transition has enabled quicker detection and differentiation of flu, COVID-19, and other respiratory pathogens outside of centralized labs. In clinical settings, advanced syndromic molecular panels—leveraging qPCR and isothermal amplification technologies like LAMP—have become the standard for simultaneous detection of multiple viruses, including HMPV and emerging coronaviruses12. New tools such as CRISPR-based diagnostics13 and next-generation sequencing (NGS)14 are further enhancing detection and surveillance, particularly for novel pathogens. Paired with AI-driven algorithms and digital platforms, these technologies are reshaping respiratory disease management—enabling faster diagnosis, more targeted treatment, and improved outbreak response15.
How Meridian Supports Respiratory Assay Development
As respiratory threats continue to evolve, assay developers face increasing pressure to deliver accurate, rapid, and adaptable diagnostic solutions that work across both clinical and decentralized settings. Meridian provides the essential building blocks to help meet these demands.
Our portfolio includes high-performance reagent solutions for molecular and immunoassay platforms, optimized for the sensitive and specific detection of respiratory pathogens. These solutions are designed for use in:
- Multiplex qPCR and LAMP assays
Meridian’s lyophilization-ready master mixes enable room-temperature stability and simplified storage - Point-of-care and at-home tests
Our specimen-specific mixes are designed for direct amplification workflows, minimizing sample prep and accelerating turnaround times - Next-generation sequencing (NGS)
We were the first to market with an ambient-temperature stable NGS sample prep kit that dramatically simplifies logistics while maintaining high sensitivity and reproducibility - Rapid immunoassays
Our portfolio includes high sensitivity paired antibodies and blocking reagents optimized for lateral flow and ELISA-based respiratory assays
Meridian’s reagents are tailored to help developers build assays that detect and differentiate between common and emerging respiratory viruses—including influenza, COVID-19, RSV, HMPV, and coronaviruses—with high sensitivity and minimal cross-reactivity. Whether you’re scaling up for syndromic panels or simplifying formats for decentralized use, our solutions support you from feasibility through commercialization.
To learn more, visit our resource page or contact us today.
参考文献:
1. History of Vaccines. (2025). 2025 flu season most intense in over a decade.
https://historyofvaccines.org/blog/2025-flu-season-most-intense-over-decade
2. Centers for Disease Control and Prevention. (2025). 2024–2025 Season Outlook – February Update.
https://www.cdc.gov/cfa-qualitative-assessments/php/data-research/season-outlook24-25-feb-update.html
3. Vergano, D. (2025). Why this year’s flu season is the worst in more than a decade. Scientific American.
https://www.scientificamerican.com/article/why-this-years-flu-season-is-the-worst-in-more-than-a-decade/
4. Vax Before Travel. (2025, March 3). Flu shots about 50% effective this season. https://doi.org/10.1021/js970135b
5. Centers for Disease Control and Prevention. (2025). 2024–2025 Seasonal Activity Summary – April Update. Centers for Disease Control and Prevention. https://www.cdc.gov/respiratory-viruses/whats-new/2024-2025-season-summary.html
6. World Health Organization. (2025). Global surveillance update on Mycoplasma pneumoniae: January–March 2025
https://www.cdc.gov/respiratory-viruses/whats-new/2024-2025-season-summary.html
7. News.com.au. (2024). The rise of metapneumovirus in China explained as outbreak spreads.
https://www.cdc.gov/respiratory-viruses/whats-new/2024-2025-season-summary.html
8. Center for Infectious Disease Research and Policy. (2024). Avian flu surge continues on US poultry farms as feds address contamination.
https://www.cidrap.umn.edu/avian-influenza-bird-flu/avian-flu-surge-continues-us-poultry-farms-feds-address-contamination-raw
9. Zhou, W., Xu, Y., Guo, H., Xu, Z., Shi, M., & Wang, L. (2024). Characterization of HKU5-CoV-2 reveals potential risk for human infection. PubMed.
https://pubmed.ncbi.nlm.nih.gov/39970913
10. Centers for Disease Control and Prevention. (2024). The challenge of emerging respiratory viruses: Global preparedness report 2024.
https://www.cdc.gov/global-health/preparedness/emerging-viruses-report.html
11. World Health Organization. (2023). Pandemic preparedness: Lessons from COVID-19 and influenza.
https://www.who.int/publications-detail/pandemic-preparedness-lessons-2023(for general preparedness, public health measures)
12. ACS Publications. (2022). CRISPR-based diagnostics: A new era for molecular testing.
https://pubs.acs.org/doi/10.1021/acssynbio.2c00496
13. Pronyk, P. M., de Alwis, R., Rockett, R., Basile, K., Boucher, Y. F., Pang, V., & Sintchenko, V. (2023). Advancing pathogen genomics in resource-limited settings. Cell Genomics, 3(12), 100443.
https://doi.org/10.1016/j.xgen.2023.100443
14. Illumina. (2023). Next-generation sequencing for infectious disease surveillance.
https://www.illumina.com/science/technology/next-generation-sequencing/infectious-disease-surveillance.html
(to support NGS tools in pathogen detection)
15. Deloitte Insights. (2024). How AI is reshaping infectious disease diagnostics.
https://www2.deloitte.com/global/en/insights/industry/life-sciences/ai-in-infectious-disease-diagnostics.html
(for AI-driven respiratory testing advances)