In antibody-based immunoassays, antigen selection is the key biological decision driving assay performance. The structure, epitope integrity, and expression format of the antigen determine how effectively antibodies are detected, and influence downstream considerations such as scalability, lot-to-lot consistency, and compatibility with formats such as ELISA, CLIA, and lateral flow.
Recombinant DNA technology, introduced in the 1980s, enabled in vitro antigen production, removing reliance on culturing live pathogens and opening the door to novel formats and otherwise inaccessible targets. One of the earliest examples was hepatitis C (HCV), where native antigen production was unfeasible due to the virus’s resistance to standard culturing. Recombinant expression of HCV core and NS3 antigens made serological testing possible and led to the first FDA-approved assays using recombinant antigens. Their value was further proven in second-generation HIV diagnostics, where recombinant gp41 and p24 replaced crude viral lysates, enhancing assay specificity and reducing false positives. Today, the decision between using native or recombinant antigens remains central to immunoassay design – shaping not only analytical performance, but also manufacturability, supply chain sustainability and clinical utility. MLS offers a wide selection of recombinant antigens for HCV.
Native Antigens in Immunodiagnostics: Maximizing Sensitivity Through Epitope Diversity
Native antigens are proteins purified directly from whole pathogens, infected cell cultures, or human tissues, preserving the full spectrum of post-translational modifications (PTMs) and conformational epitopes found in vivo. Their structural fidelity makes them especially valuable in immunodiagnostics for pathogens like cytomegalovirus (CMV), which express distinct antigens at different stages of infection and trigger broad, polyclonal antibody responses. Because individuals generate antibodies to multiple viral proteins in varying amounts, native antigens more accurately reflect the true antigenic landscape – enhancing the likelihood of detecting clinically relevant antibodies that may be missed by linear or recombinant-only formats.
While native antigens offer important biological advantages, their use also presents practical challenges. Key advantages and limitations include:
Advantages:
- Preserves native structure and PTMs, enabling recognition of conformational epitopes critical for detecting clinically relevant antibodies missed by linear or misfolded antigen
- Enables broad antibody detection by presenting full epitope diversity, increasing sensitivity and detection across diverse populations and immune backgrounds
- Broad epitope diversity enables detection of antibodies across all stages of infection – critical for pathogens like HSV, Rubella, and Toxoplasma gondii, where stage-specific immune responses require comprehensive antigen coverage
Limitations:
- Subject to variable yield and quality due to dependence on biological source material, impacting consistency.
- Difficult to scale for manufacturing, especially when sourcing low-abundance or unculturable pathogens.
- Involves biosafety or ethical risks when derived from infectious agents or human tissues, limiting accessibility and use.
Meeting Diagnostic Demands: The Strategic Role of Recombinant Antigens
Recombinant antigens are produced by expressing defined gene constructs in host systems such as E. coli, yeast, insect, or mammalian cells. This enables precise design control over antigen composition—allowing inclusion of key antibody-binding regions, exclusion of cross-reactive or homologous sequences, and fine-tuned incorporation or exclusion of structural features such as site-specific post-translational modifications (PTMs). Yields can be optimized, lot-to-lot consistency is easier to maintain, and reliance on native source material is eliminated, reducing risk and enabling scalable, controlled manufacturing. However, while these systems offer design flexibility, native-like PTMs which can be essential for accurate epitope presentation in some assays, are typically only replicated in mammalian or insect expression systems.
Beyond scalability, a key strategic advantage of recombinant antigens is their modularity. Developers can selectively express specific immunogenic domains or fragments to optimize assay performance. For example, in CMV (which elicits a broad, polyclonal immune response) recombinant antigens can enhance assay specificity by targeting low-abundance or stage-specific antigens that may trigger weak or variable antibody responses across individuals. This targeted approach can support earlier detection and improve overall clinical accuracy.
In many cases, recombinant antigens are not just advantageous but essential. Some pathogens cannot be sourced natively due to biological, safety, or ethical constraints. High-risk agents such as HIV and Ebola require high containment facilities, making native antigen production impractical or hazardous. Others, such as Treponema pallidum (syphilis), extremely difficult to culture, necessitate the use of recombinant antigens like TpN15, TpN17, and TpN47 to support serological assay development. For example, Meridian offers more than a dozen TP antigens – all of them recombinant. In autoimmune diagnostics, antigens such as thyroglobulin are available in both native and recombinant formats, but the native form is typically derived from human tissue and is difficult to source—raising challenges around reproducibility, ethical considerations, and long-term supply stability. As demand for earlier and more precise detection grows, recombinant antigens offer a scalable solution when traditional sources are limiting.
While recombinant antigens address many sourcing and performance challenges, they also present specific strengths and limitations that must be considered in the diagnostic context:
Advantages:
- Deliver high lot-to-lot consistency and purity, reducing variability in assay performance
- Scalable, animal-free production avoids biosafety and ethical sourcing constraints
- Allow targeted epitope selection to improve specificity and minimize cross-reactivity
- Support multiplexing and POC test design with custom antigen formats and stability profiles
Limitations:
- May lack native PTMs unless expressed in mammalian or insect systems, potentially affecting antibody recognition
- Risk of misfolding in prokaryotic systems, which can compromise epitope integrity and reduce binding efficiency
- May show reduced sensitivity if key conformational epitopes are absent or improperly presented
Hybrid Antigen Strategies: Enhancing Diagnostic Performance Through Complementary Formats
While recombinant antigens offer clear advantages in cross-reactivity control – enabling the selective expression of non-conserved or immunodominant regions to improve specificity – they may not fully represent the native antigenic landscape. Linear epitopes alone can miss conformational or post-translationally modified structures essential for antibody recognition, potentially limiting sensitivity. To overcome these challenges, many FDA-approved assays adopt a hybrid strategy that combines native and recombinant antigens to balance sensitivity and specificity.
This strategy is exemplified in fourth-generation HIV tests, which pair recombinant gp41 with native p24 (Meridian offers both of these) to enhance early detection while maintaining confirmatory accuracy. Lyme disease (Borrelia) assays often combine recombinant VlsE with native flagellar proteins to capture immune responses across different disease stages. TORCH panels, including Toxoplasma, Rubella, CMV, and HSV, frequently incorporate native antigens to preserve structural epitope integrity, while recombinant components help reduce cross-reactivity and improve consistency, particularly in HSV and CMV testing. Used together, native and recombinant antigens expand epitope coverage, reduce false negatives, and improve diagnostic sensitivity across diverse patient populations by capturing broader immune responses.
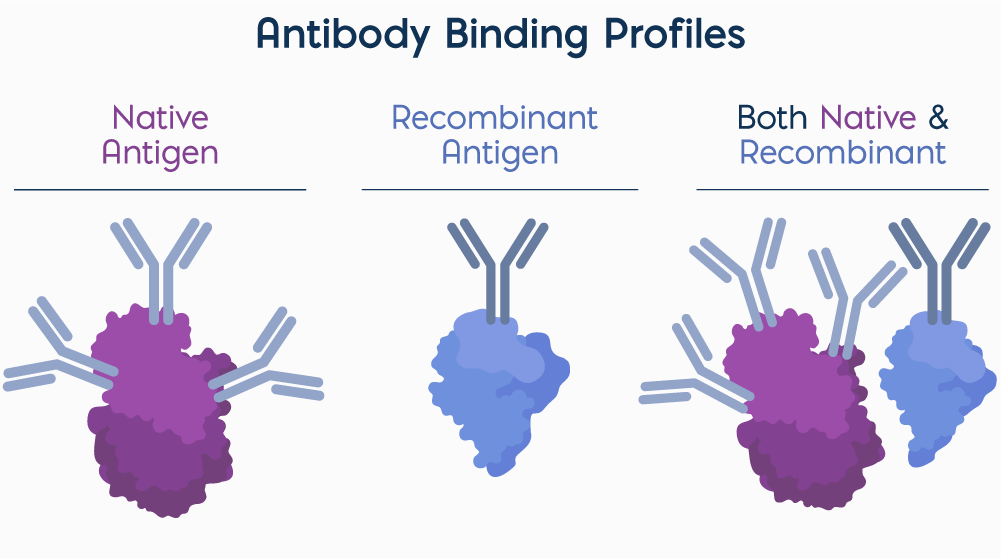
Native antigens present a diverse array of conformational and linear epitopes, enabling recognition by multiple antibodies or multiple binding sites on a single antibody. In contrast, recombinant antigens are typically designed to display a defined epitope or domain, offering high specificity for targeted antibody detection. Combining native and recombinant antigens allows assays to capture a broader range of antibody responses while maintaining specificity for clinically relevant targets.
Your Reliable Source for Native and Recombinant Antigens at Scale
Whether you need the biological fidelity of native proteins or the design flexibility of recombinant formats, Meridian Bioscience offers validated antigens and expert support across every stage of assay development to meet your reagent needs. Both our native and recombinant antigens are manufactured under stringent quality controls to ensure lot-to-lot consistency, minimizing revalidation risk and supporting regulatory confidence. We maintain multiple lots in inventory to facilitate application testing, lot bridging, and long-term reservation, helping you move efficiently from feasibility to scale. With decades of experience in immunodiagnostics, Meridian is your trusted partner in building robust, commercially viable diagnostics.
Explore our full portfolio of native and recombinant antigens to support your next diagnostic innovation.