Point-of-care (POC) diagnostics are at the forefront of modern healthcare, offering rapid and convenient solutions for early disease detection and monitoring. A recent significant advancement in POC is the integration of saliva tests, which have the potential to make POC testing even more accessible and efficient. Saliva, often called the “mirror of the body,” contains a range of biomarkers, including DNA, RNA, proteins, and metabolites, making it a suitable specimen for a range of diseases including respiratory infections, STDs, tropical diseases, hormone disorders and cancer.
The Point-of-Care (POC) Revolution
POC diagnostics are assays that can be conducted outside a traditional laboratory setting, often at or near the patient’s location. These tests are designed to be rapid, user-friendly, and capable of delivering results within 10 to 20 minutes. Sample collection plays a significant role in the accuracy and reliability of POC tests and many sample types such as blood, nasal or throat swabs, require a trained professional to collect and can cause patient discomfort and pain. Saliva, in contrast, is simple to obtain and can be self-sampled, offering better testing accessibility and lower testing costs. As a result, saliva is gaining attention in the field of remote POC and home-based testing across a wide range of disease targets.
The Power of Saliva in POC Diagnostics
Until a few years ago, commercial saliva tests were only available for hormone testing, HIV, and drug/alcohol assays. One of the technological barriers faced with developing a salivary diagnostic has been assay sensitivity due to the low concentrations of analytes found in saliva compared to blood (100- to 1000-fold lower)1 and the high concentration of PCR and other assay inhibitors. However over the past decade, technological advances such as saliva-specific molecular master mixes, and innovative saliva collection devices that efficiently capture a specified volume of saliva and reduce the risk of insufficient samples or poor sample quality, have improved the usability of saliva specimens for diagnostic testing.
During the pandemic, there was a significant need for widespread population testing in order to control the spread of SARS-CoV-2. Consequently, saliva was highly sought after as an alternative specimen to nasopharyngeal swabs, and several studies proved its effectiveness when used in traditional SARS-CoV-2 PCR tests and in at-home rapid antigen tests2. In addition, research found that for repeat testing, saliva samples were more likely to be more acceptable to patients than nasopharyngeal swabs3.
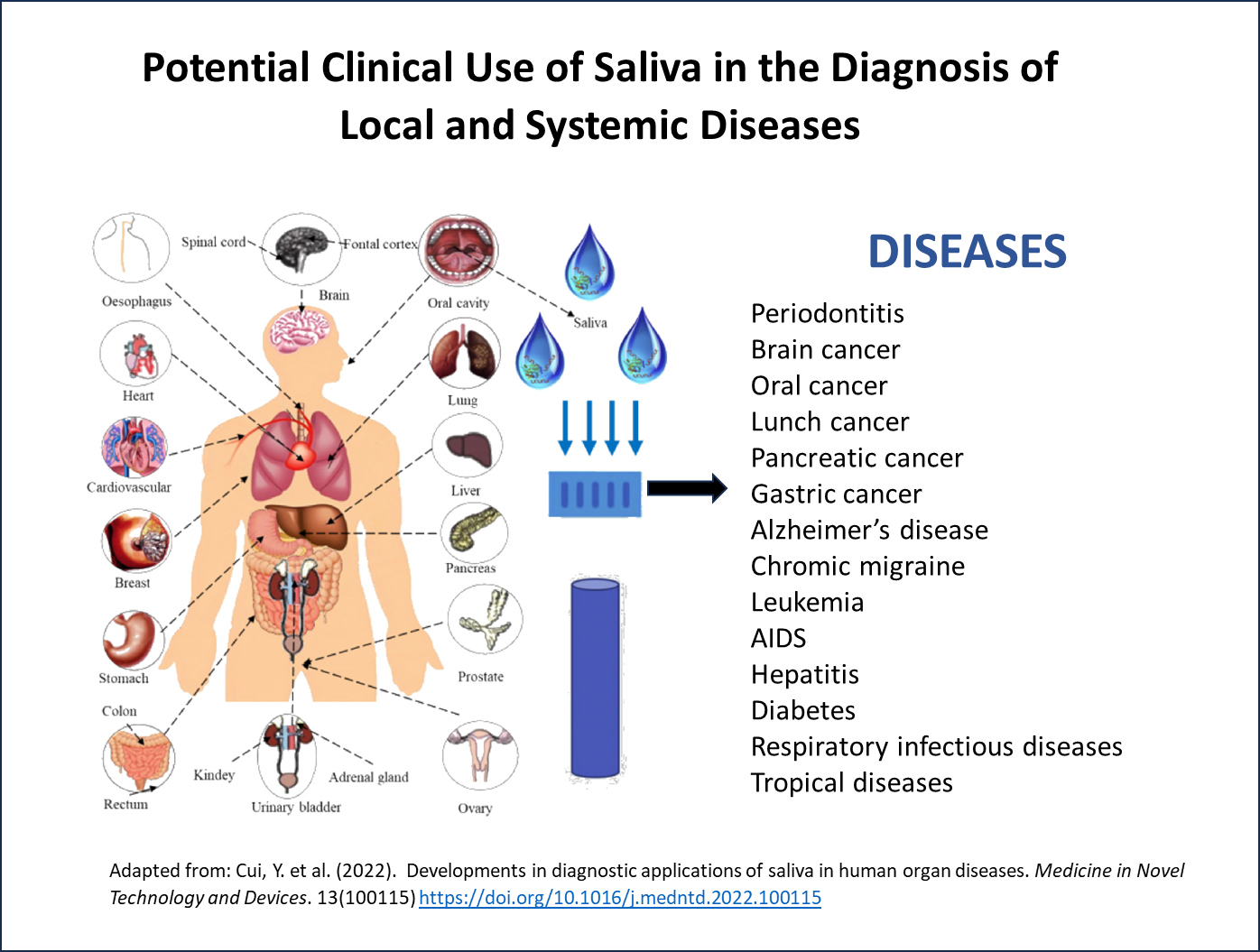
Since the pandemic, using saliva has become more mainstream, and in vitro diagnostic companies are showing its utility in high-throughput instruments4 and in the detection of a range of diseases including tropical diseases5, heart disease, human papillomavirus (HPV)-related cancers, breast cancers, lung cancers, as well as monitoring treatment efficacy, detecting disease reoccurrence, and stratifying patient risk. Saliva testing is even being researched for the detection of new biomarkers such as tau protein for Alzheimer’s disease4.
Saliva-Specific Mixes in Point-of-Care (POC) Diagnostics: A Game-Changer
POC diagnostics are at the forefront of a transformative shift towards faster and more accessible testing and saliva as a testing specimen is more user-friendly, more cost-effective, and can deliver faster collection-to-results than traditional matrixes such as blood. However, the challenges in using saliva as a sample type include ensuring sample quality, preventing contamination, and achieving efficient extraction methods despite the lower DNA and RNA concentrations. For molecular assays in particular, saliva has been a challenge due to the enzyme inhibitors inherently present, and several technologies have focused on improving DNA and RNA extraction techniques to minimize the carryover of inhibitors. Alternative technologies have focused on developing inhibitor-tolerant mixes that enable the direct detection from saliva to help minimize sample loss, increase assay testing-turn-around time, and minimize the potential for contamination.
Meridian’s Specimen-Specific™ Direct Saliva Master Mixes are one the most advanced chemistries available designed for direct detection assays from saliva samples. These mixes are optimized for sensitive and robust performance using crude saliva and are ready to use, only requiring the addition of assay-specific primers and probes. In addition, they are formulated with excipients for downstream lyophilization or air-drying to create ambient temperature stable assays which are ideal for POC applications. In comparison studies, Meridian’s Saliva-Specific Master Mixes demonstrated their significant performance advantages in efficiently amplifying RNA and DNA from extracted samples and crude saliva samples, even after the mixes had been dried down and briefly stored at room temperature.
As saliva specimens for diagnostic testing expand into new disease areas, saliva-specific master mixes for molecular assays will be a game-changer, enabling assay developers quicker development times, simpler, extraction-free workflows, and greater assay performance in terms of sensitivity and inhibitor tolerance. To learn more about Meridian’s Specimen-Specific™ Direct Saliva mixes for PCR and LAMP applications, visit: https://www.meridianbioscience.com/lifescience/products/molecular-reagents/specimen-specific-inhibitor-tolerant-master-mixes
References:
- Cui, Y. et al. (2022). Developments in diagnostic applications of saliva in human organ diseases. Medicine in Novel Technology and Devices. 13(100115) https://doi.org/10.1016/j.medntd.2022.100115
- Duncan, D.B. et al. (2023). Performance of saliva compared with nasopharyngeal swab for diagnosis of COVID-19 by NAAT in cross-sectional studies: Systematic review and meta-analysis. Clin Biochem. 117:84-93. https://doi:10.1016/j.clinbiochem.2022.08.004
- Covid-19: Concerns persist about purpose, ethics, and effect of rapid testing in Liverpool (2020) BMJ. 371:m4690 https://doi.org/10.1136/bmj.m4690
- Michaud, S. (2022). Saliva in the Spotlight. Clinical Laboratory News. Retrieved Oct 19, 2023 from: https://www.aacc.org/cln/articles/2022/janfeb/saliva-in-the-spotlight
- Diaz, J., et al. (2023). A mixed methods study assessing the adoption potential of a saliva-based malaria rapid test in the Democratic Republic of Congo. Malar J.;22(1):180. https://doi: 10.1186/s12936-023-04599-y-