AMPLIFYING PRECISION
High-Quality Reagents for Multiplex Respiratory Assays
Point-of-Care (POC) Respiratory Testing
Point-of-care diagnostics for respiratory diseases has been a rapidly evolving field and the COVID-19 pandemic helped to accelerate the development and public uptake of point-of-care respiratory testing. Both immunodiagnostics on paper platforms and molecular diagnostics on microfluidic platforms have received particular attention for the development of POCT techniques (Science Direct LTD). However, developing POC multiplex assays for respiratory diseases is particularly challenging due to the potential for cross-reactivity between pathogens and the high sample complexity, with respiratory specimens often containing various types of cells, mucus, and other components which can interfere with the test, and affect assay accuracy.
Respiratory tract infections are the most common diseases affecting humans and the global respiratory pathogen testing market is expected to grow from US$3.1 billion in 2022 to US$4.2 billion by 2030. With the ongoing evolution of healthcare diagnostics to improve patient care and outcomes, POC testing methods for respiratory diseases is pivotal in facilitating early detection and effective management.
Meridian offers comprehensive solutions to meet your needs with both reagent solutions for rapid immunoassays and molecular tests using either qPCR or LAMP techniques. to meet your needs.
Diagnostic Assay Solutions for Top 3 Viral Infections
Influenza A/B
Influenza viruses are RNA viruses that are prone to antigenic drift and reassortment, which enables new strains of the virus to emerge each year. Diagnostic influenza tests include rapid influenza diagnostic tests (RIDTs), direct fluorescent antibody
stains, viral cultures, and molecular assays. The tests typically distinguish influenza type A from
influenza B, and can also identify influenza A subtypes. Rapid assays for influenza typically detect the nucleoprotein (NP) using swabs or samples of secretions taken from a patient’s nose or throat and the entire test can be performed at the point-of-care (POC). The results are rapid (under 30 minutes) however they may not be able to indicate which specific strain of flu the patient has. Rapid molecular assays are the preferred diagnostic tests because they can be done at the point of care, are highly accurate, and have fast results.
Read More
SARS-CoV-2
SARS-CoV-2 is a coronavirus responsible for the 2020 COVID-19 pandemic. Current diagnostic tests for the SARS-CoV-2 include molecular tests, antigen tests and serological tests. Molecular tests to detect viral nucleic acids are the ‘gold standard’ for detecting SARS-CoV-2 infection, but they can be time-consuming and generally require a laboratory or trained personnel. Immunoassays are less sensitive, but they offer rapid results. Most antigen detection assays use antibodies that recognize SARS-CoV-2 nucleocapsid protein as it is the most abundant viral protein. Spike protein has also been targeted, especially in assays that use alternative sample types such as saliva or nasal swabs that can be collected by an individual. The antibodies detect a conserved region N protein and they do not exhibit any cross-reactivity to influenza A/B, RSV or seasonal coronavirus strains.
Read More
RSV
Respiratory Syncytial Virus (RSV) is a common respiratory virus that usually causes mild, cold-like symptoms but can cause more serious problems such as bronchiolitis and pneumonia in babies and young children. RSV virus consists of three main proteins and the fusion protein (which is responsible for fusion to the host membrane) is the current leading target for diagnostic assays and the majority of vaccines and immunotherapies under development. Several different types of laboratory tests are available for the diagnosis of RSV infection including ELISA, rapid lateral flow, Direct Fluorescent Antibody Detection (DFA), neutralization assay and RT-PCR. Most clinical
laboratories currently utilize EIA antigen detection tests, and many supplement antigen testing with cell culture or immunofluorescence assays to confirm diagnosis.
Read More
Meridian’s Portfolio of Blocker Solutions

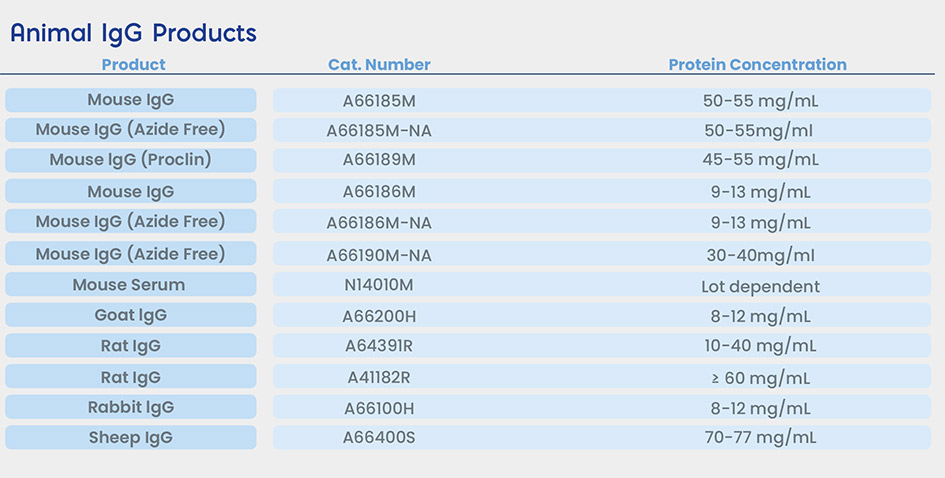
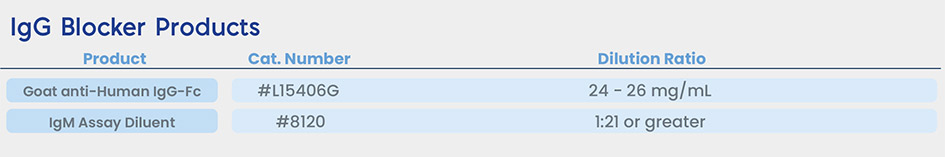
Certificate of Analysis (COAs)
Proven Results Using TRU Block™
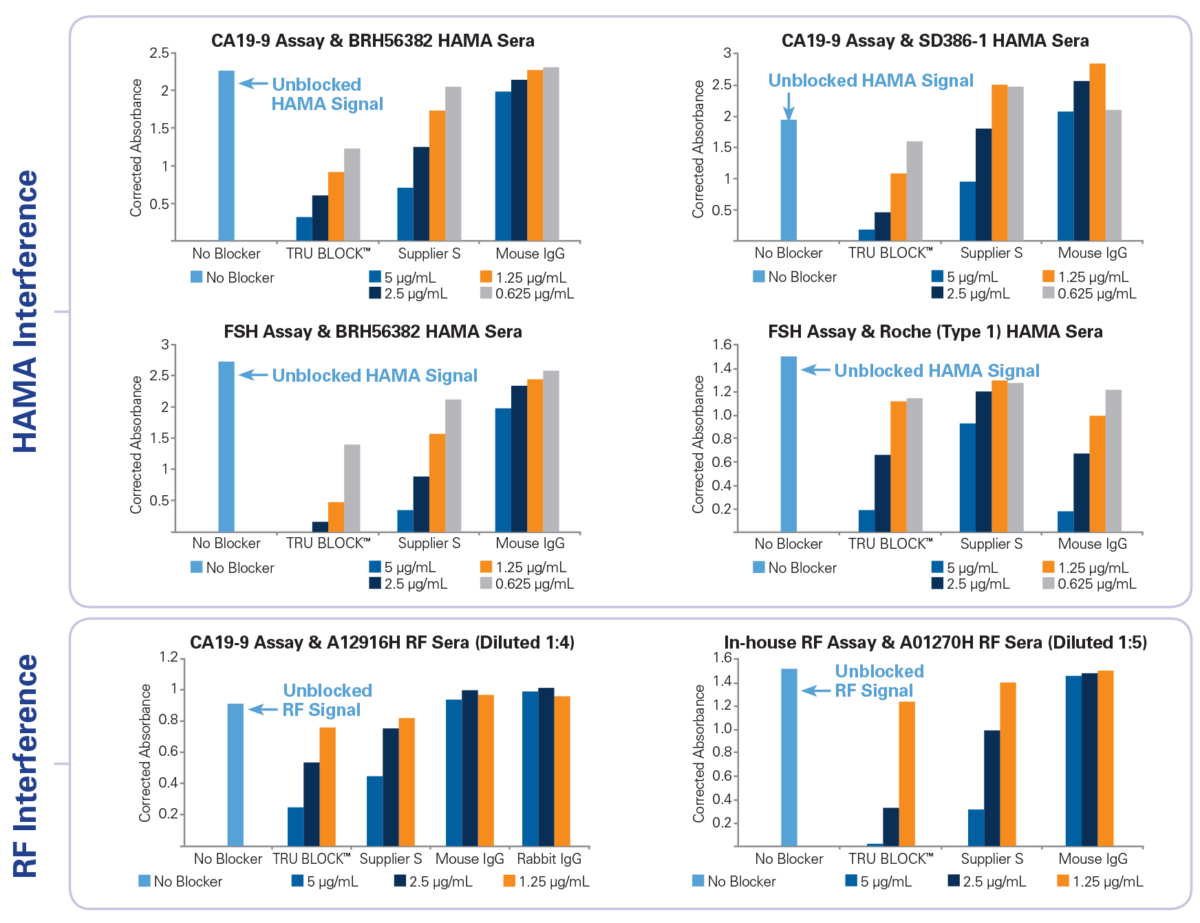
Three different double mouse monoclonal sandwich assays (a commercial CA19-9, a commercial FSH and an in-house RF assay) were used to compare the performance of TRU Block™ against mouse IgG and supplier S active HAMA blocker. To quantify the relative amount HAMA or RF activity in each human sera sample within an assay, the assay was performed as per the manufacturer’s instructions using a sample diluent buffer containing no mouse IgG or other blocker. Blocking solutions containing either TRU Block™, mouse IgG or supplier S blocker were prepared using the same sample diluent buffer at four different concentrations (5 μg/mL, 2.5 μg/mL, 1.25 μg/mL and 0.625 μg/mL). The effectiveness of each blocker was determined by the relative suppression of HAMA or RF signal (i.e. comparing the absorbance of samples with no blocker to those with various blockers). Native HAMA positive sera samples were obtained from Scantibodies, Inc. (SD386-1) and BioReclamation (BRH56382). Another sample was obtained from Roche (Roche Type 1) which contains pooled normal human serum spiked with Roche proprietary HAMA. RF sera samples (MLS Cat No. A12916H and A01270H) were obtained internally and are available for purchase from Meridian’s product catalog (www.meridianbioscience.com/lifescience). The study results demonstrate that TRU Block™ is equivalent to or better than a supplier S active blocker on all HAMA and RF sera samples tested.
Resources to Learn More

Learn More
Lead about the factors that need to be considered in selecting the right blocker for an immunoassay.

Watch a Short Video
Find out how TRU Block™ works to prevent heterophilic antibody interference.
Read Whitepaper
Gain a deeper understanding of the challenges faced by IVD manufacturers by reading the whitepaper “Reducing Errors in Immunoassay Testing”.
FAQs: Immunoassay Interference Blockers
Immunoassays used for human in vitro diagnostics (IVD) typically use animal-derived antibodies to recognize specific disease markers. Some patients have antibodies in their blood that can react with animal antibodies in the immunoassay and cause a false result. Although the frequency of these interferences is low, false-positive results have a significant negative impact on the quality and competitiveness of a diagnostic assay as well as on the lives of those individuals who have been falsely diagnosed.
Human anti-mouse antibodies (HAMA) is the most well-known antibody interference due to the wide use of mouse monoclonal antibodies in diagnostic applications. Mouse IgG can block HAMA found in patient serum.
No. HAMA only represents one type of heterophilic antibody (HA) interference – others include HA to animals such as goat (HAGA), sheep (HASA), and rabbit (HARA) which can cause false results when antibodies originating from these animals are used in immunoassays. In addition to HA there is another class of interference called Rheumatoid factor (RF), which is an autoantibody that reacts with the patient’s own immunoglobulin (Ig) and can cross react with animal Ig, similar to HA/HAMA interference.