Lyo-Ready Direct DNA and RNA/DNA qPCR Urine Master Mixes
Lyo-Ready Direct DNA qPCR and RNA/DNA qPCR Urine mixes are glycerol-free mixes that contain optimized excipients compatible with lyophilization. The mixes have been designed for the development of quantitative PCR urine test assays for direct detection of DNA and RNA from crudely processed human urine specimens.
Can also be used wet and/or with purified nucleic acid
Have questions about a product?
Contact us to learn more about Meridian’s molecular or immunoassay reagent portfolio. We want to hear from you!
Lyo-Ready Direct DNA qPCR Urine, 4x, MDX152
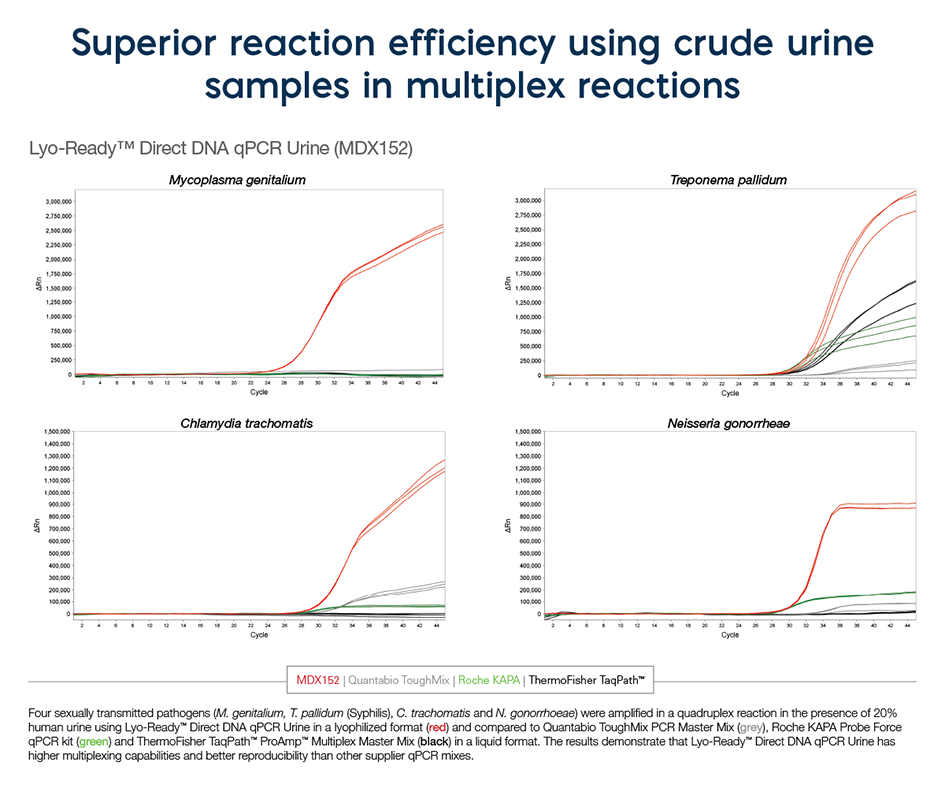
Inhibitor tolerance to 20% urine. Perfect for multiplexing and fast cycling conditions. Used in the detection of urinary track infections, STD, tropical diseases, cancer screening, bladder and prostate cancer.
Documents & Resources
Lyo-Ready Direct RNA/DNA qPCR Urine, 4x, MDX153
Inhibitor tolerance to 20% urine. Perfect for multiplexing and fast cycling conditions. Used in the detection of urinary track infections, STD, tropical diseases, cancer screening, bladder and prostate cancer.
Documents & Resources
Urine is an ideal clinical specimen because it is excreted in large quantities, is non-invasive, and it can be self-sampled. Urine specimens are used in the diagnosis and management of infectious diseases, bladder cancer and urinary tract infections (UTIs). However, urine contains substances such as urea and nucleases that can damage DNA or inhibit the PCR reaction.
Meridian’s new Lyo-Ready Direct Urine molecular mixes are unique in that they enable highly sensitive, very fast detection of target DNA or RNA directly from high concentrations of urine. With the CDC reporting a 30% increase in reportable sexually transmitted disease (STDs) between 2015 and 2019, these direct urine molecular mixes are ideal, as they allow less invasive sample collection (than a cervical or urethra swab) and encourage routine screening. They have been formulated specifically to overcome the inhibitors found in urine – no further optimization is required aside from the addition of primers and probes. Furthermore, these mixes can be used in a wet format or dried down by lyophilization to create ambient-temperature stable assays.
Catalogs & Brochures
Lyo-Ready Direct DNA and RNA/DNA qPCR Urine Master MixesLyo-Ready Direct DNA and RNA/DNA qPCR Urine Master Mixes
Lyo-Ready LAMP Mix, 4xLyo-Ready LAMP Mix, 4x
Lyo-Ready RT-LAMP 1-Step Mix, 4xLyo-Ready RT-LAMP 1-Step Mix, 4x
Air-Dryable Direct Urine CatalogAir-Dryable Direct Urine Catalog
FAQs: qPCR Urine Test
The advantages of a quantitative PCR urine test are that it is completely non-invasive, and it can be self-sampled in large quantities. Urine testing is used in the diagnosis and management of infectious diseases (including STDs), urinary tract infections (UTIs) and bladder cancer.
The most common infections are urinary tract infections (UTIs), most commonly E. coli, P. mirabilis, Enterococcus, Staphylococcus and Klebsiella. This is followed by sexually transmitted diseases (STDs) such as syphilis, gonorrhoea, chlamydia, trichomoniasis, hepatitis B (HBV), herpes simplex virus (HSV), HIV and human papillomavirus (HPV). Several other infections can be diagnosed by urine DNA and urine RNA tests including community-acquired pneumonia (CAP), legionellosis, tuberculosis, congenital cytomegalovirus (CMV) infection, and arboviruses, such as Chikungunya virus, dengue virus and Zika virus. Parasites can also be diagnosed from urine testing for the detection of eggs from Schistosoma haematobium.
Cancer cells or their DNA or RNA are too big to pass through the kidneys and will have to come from the bladder or ureters, so bladder cancer is the most obvious urine cancer, however prostate and cervical cancer are also detectable.
We have used up to 25% human urine with our mix.
It is stable for up to 2 years at ambient temperature if correctly stored in sealed pouches.
That will depend on the bacteria, for some bacteria you may not need to do any pre-treatment, however some may require heating to 95°C for 5 min and for some tougher bacteria, a Proteinase K step (Proteinase K 55°C for 15 min and then at 98°C for 5 min) or using a non-ionic detergent (such as Tween-20 to final concentration of 0.2% followed by incubation at room temperature for 10 min) may be necessary.
We do not see a performance difference between the two mixes, which is why we call the Lyo-Ready Direct RT-qPCR Urine mix – Lyo-Ready Direct RNA/DNA qPCR Urine mix, as it can be used for both.
Yes, the Lyo-Ready Direct DNA qPCR Urine and Lyo-Ready Direct RNA/DNA qPCR Urine can be used as a liquid mix or lyophilized and stored for up to 24 months without needing to change the reaction conditions and lyophilization/ambient temperature storage will not affect the sensitivity of the test.
Get In Touch With A Specialist
Have questions about a product? Want to learn more about Meridian’s molecular or immunoassay reagent portfolio? We want to hear from you!