Lyo-Ready™ Direct DNA and RNA/DNA qPCR Stool
Lyo-Ready™ Direct DNA qPCR and RNA/DNA qPCR Stool mixes are glycerol-free mixes that contain optimized excipients compatible with freezing and lyophilization and they are designed for the direct molecular quantitation of DNA and RNA from crudely processed stool specimens.
Can also be used wet and/or with purified nucleic acid
Have questions about a product?
Contact us to learn more about Meridian’s molecular or immunoassay reagent portfolio. We want to hear from you!
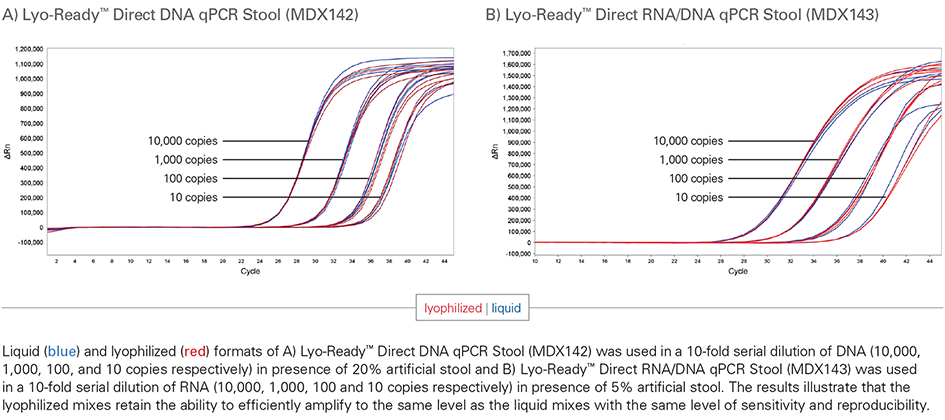
Sensitive Detection in up to 20% Stool
Lyo-Ready™ Direct DNA qPCR Stool, MDX142
Available in 5 mL (1,000 Rxns) or 50 mL (10,000 Rxns) aliquots
Lyo-Ready™ Direct RNA/DNA qPCR Stool, MDX143
Available in 5 mL (1,000 Rxns) or 50 mL (10,000 Rxns) aliquots
PCR inhibitors found in stool specimens, such as bile salts, polysaccharides, hematin and catabolic substances, have posed a big challenge to developing assays that can directly amplify DNA or RNA. In addition, due to the high complexity and heterogeneity of fecal matter, sample preparation has traditionally been cumbersome, requiring the removal of possible interfering substances such as food debris, desquamated epithelial cells and mucus from the specimen. Lyo-Ready™ Direct DNA qPCR and RNA/DNA qPCR Stool mixes are designed for the direct qPCR/RT-qPCR analysis, requiring minimal stool sample processing. The mix contains an optimized blend of additives to negate inhibitors while maintaining the quality and integrity of the patient sample. As the need for fast, non-invasive testing for gastrointestinal conditions increases, short turn-around times, higher sensitivity and a long shelf-life become important distinguishing features.
If you are developing an infectious disease assay for parasites, viruses, bacteria, or a screening assay for inflammatory bowel disease or gastric or colon cancer, Lyo-Ready™ Direct DNA qPCR and RNA/DNA qPCR Stool mixes will speed up your assay development time. The mix only requires the addition of primers and probes to create a liquid or ambient-temperature stable assay that can be dried in a laboratory oven, avoiding the need for expensive lyophilization.
Catalogs & Brochures
Lyo-Ready Direct DNA and RNA/DNA qPCR StoolLyo-Ready Direct DNA and RNA/DNA qPCR Stool
Air-Dryable MixesAir-Dryable Mixes
AIR-DRYING MOLECULAR ASSAYSAir-Drying Molecular Assays
Air-Dryable Direct BloodAir-Dryable Direct Blood
FAQs: qPCR Stool Test
A quantitative PCR stool test can allow identification of enteric pathogens such as harmful bacteria, fungi, viruses, or parasites down to the genus or species level, fast and accurately in a few hours, rather than the culture-based stool testing that can take several days. It can also be used as a non-invasive method for screening autoimmune conditions and cancer.
The fecal occult blood test (FOBT) has been the most widely used gastrointestinal immunoassay to screen colorectal cancer (CRC) for over three decades, however, recent large-scale studies have shown that the FOBT lacks the sensitivity to detect early stages of CRC. A qPCR cancer stool test is a better diagnostic tool as it offers significant improvement in sensitivity and accuracy and can be used to screen for multiple targets and so speed up diagnosis, therefore improving prognosis.
We have used up to 25% human stool with our mix, this is homogenized, and clarified by centrifugation and some pre-treatment may be required.
There are several buffers on the market that can be used for stool testing, they include phosphate-buffered saline (PBS), Cary-Blair Medium, Stool Transport and Recovery (STAR) buffer and DNA/RNA Shield.
It is stable for up to 2 years at ambient temperature if correctly stored in sealed pouches.
Yes, most of it comes from cells that are sloughed into the lumen after cells die and are replaced, these cells go through apoptosis, releasing the DNA. Tumor cells may be present, but they will normally be less than 1% of the total human DNA fragments present in the overall stool sample.
Pre-treatment will depend on what you are looking at, cfDNA may not need pre-treatment, but you may need to heat to 95°C for 5 min and for some bacteria, a Proteinase K step (Proteinase K 55°C for 15 min and then at 98°C for 5 min) or using a non-ionic detergent (such as Tween-20 to final concentration of 0.2% followed by incubation at room temperature for 10 min) may be necessary.
We do not see a performance difference between the two mixes, which is why we call the Lyo-Ready™ Direct RT-qPCR Stool mix – Lyo-Ready™ Direct RNA/DNA qPCR Stool mix, as it can be used for both.
Yes, the Lyo-Ready™ Direct DNA qPCR Stool and Lyo-Ready™ Direct RNA/DNA qPCR Stool can be used as a liquid mix or lyophilization and stored for up to 24 months without needing to change the reaction conditions and lyophilization/ambient temperature storage will not affect the sensitivity of the test.
Get In Touch With A Specialist
Have questions about a product? Want to learn more about Meridian’s molecular or immunoassay reagent portfolio? We want to hear from you!