Cancer diagnostics have undergone a major transformation over the past two decades, shifting from late-stage diagnosis and generalized treatment toward earlier detection, molecular stratification, and personalized therapy. With this shift comes the need to detect clinically actionable biomarkers at increasingly low concentrations, while also supporting broader mutational profiling to expand access to molecularly matched therapies and guide more precise treatment decisions.
As the oncology diagnostics landscape continues to evolve — supporting earlier detection, real-time monitoring, companion diagnostics and decentralized testing – quantitative PCR (qPCR) remains a foundational tool. Its combination of high analytical sensitivity, rapid turnaround time and cost-efficiency makes it uniquely suited for informing therapeutic decision-making at scale, especially in time-sensitive or resource-constrained settings.
What Sets qPCR Apart in Oncology Diagnostics?
qPCR’s strong multiplexing capability allows multiple clinically relevant mutations to be detected in a single reaction, without compromising sensitivity or speed. This makes it particularly well-suited for oncology applications where actionable targets span several genes, and sample material is scarce. For example, in non-small cell lung cancer (NSCLC), multiplexed qPCR panels can simultaneously assess alterations in EGFR, KRAS, BRAF and ALK – delivering results faster and using less input material than sequential or panel-based next-generation sequencing (NGS) approaches. Solutions such as Biofidelity’s Aspyre Lung Reagents1 and the AmoyDx® Pan Lung Cancer PCR Panel2 showcase the strong clinical potential of multiplexed qPCR, with ongoing advancements expected to further expand regulatory clearance and clinical adoption. This makes qPCR an attractive option for labs aiming to expand molecular coverage without the complexity, cost or turnaround time associated with sequencing platforms. It is especially useful in tissue-limited cases such as fine needle aspirates or cell-free DNA (cfDNA) from liquid biopsies, where maximizing data from minimal input is critical.
Fast Results with Minimal Infrastructure
Unlike sequencing platforms, which can take days to generate and analyze data, qPCR delivers clinically actionable results within hours. This rapid turnaround is especially valuable in time-sensitive scenarios such as selecting targeted therapies or enrolling patients into mutation-driven clinical trials where quick decision-making is critical.
qPCR is also highly scalable and automation-friendly, supporting high-throughput testing without the need for significant capital investment or complex infrastructure. Its compatibility with standardized 96- or 384-well formats makes it ideal for a wide range of settings, from centralized reference labs to hospital-based molecular laboratories and decentralized or resource-limited environments.
In addition, qPCR assays are generally easier to interpret, validate and implement within regulatory frameworks compared to more complex methods like next-generation sequencing (NGS).
Cost-Effective for Scalable Cancer Screening
While sequencing technologies have deepened our understanding of the cancer genome, qPCR remains a significantly more cost-effective option for targeted mutation detection. Test costs typically range from $50 to $200 – substantially less than the $300 to $3,000 price range of NGS.
This affordability makes qPCR especially well-suited for large-scale screening initiatives and routine clinical diagnostics, particularly in resource-conscious healthcare systems. For example, in India, qPCR serves as a cornerstone of HPV-based cervical cancer screening3, while in China, it underpins EGFR mutation testing across both major cancer centers and regional hospitals4.
With its unique combination of speed, cost-efficiency and scalability, qPCR has become a practical and widely deployable tool for population-scale cancer screening – expanding access to precision diagnostics without compromising analytical accuracy.
Key innovations in qPCR Chemistry include:
- Inhibitor Resistance: Next-generation polymerases and buffers are engineered to tolerate PCR inhibitors commonly found in clinical matrices, such as heparinized plasma, whole blood or FFPE-derived nucleic acids.
- Thermal Stability: Enzymes now withstand higher-temperature, faster-cycling protocols without loss of activity – enabling faster run times and greater assay reliability.
- Lyophilization Compatibility: Ambient-temperature stable formulations support cold chain–independent transport and storage – ideal for decentralized testing or OEM applications.
- Multiplexing Efficiency: Advanced master mixes and probe systems enable detection of multiple mutations in a single reaction – critical for cancers with complex mutational profiles.
Together, these advances have significantly broadened qPCR’s clinical utility—enhancing sensitivity, specificity and throughput — while enabling more reliable and efficient quantification of nucleic acids across a range of sample types and conditions.
Meridian’s Reagent Solutions for Oncology Assay Developers
Meridian Bioscience offers a portfolio of qPCR and RT-qPCR reagents designed specifically for clinical and oncology applications. These include high-performance master mixes, custom-formulated master mixes and ambient-stable kits, for scalable diagnostic deployment.
Product Highlights:
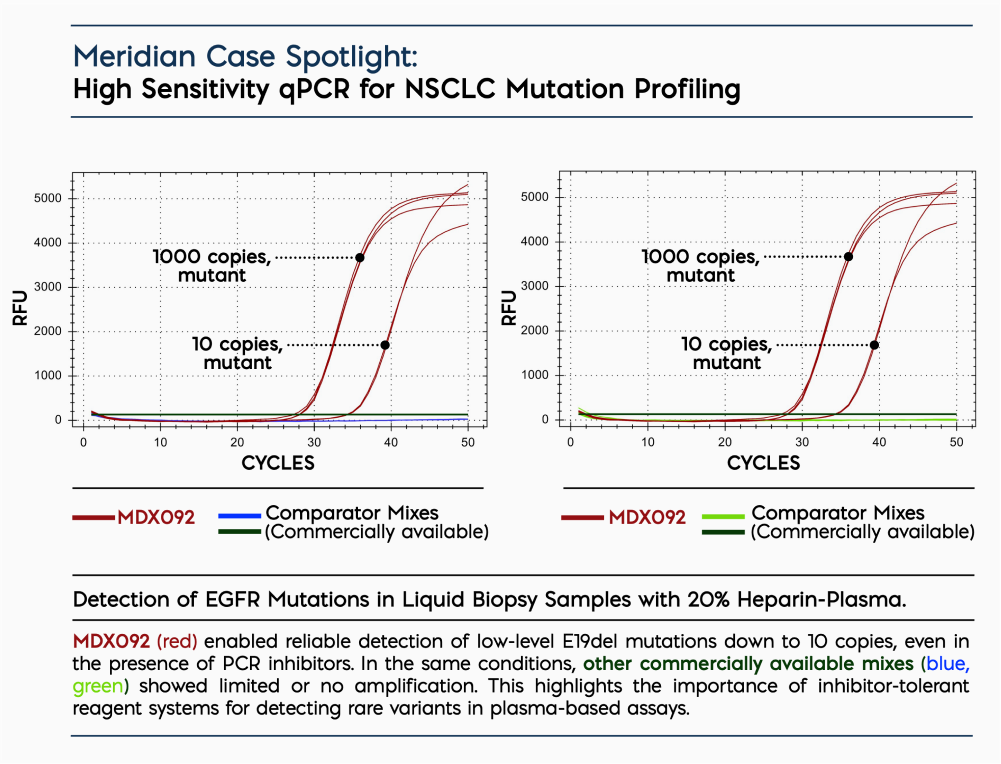
- Robust Performance in Clinical Matrices: Engineered for plasma, FFPE, cfDNA and low-input samples.
- High Sensitivity: Consistently detects low-frequency variants at <0.1% VAF.
- Ambient-Stable Options: Reduce cold chain costs and support global distribution.
- Custom OEM Services: Tailored formulations for regulatory alignment or diagnostic integration.
With a focus on workflow efficiency and clinical robustness, Meridian’s reagents empower oncology developers to build assays that meet the speed, sensitivity and scalability required in modern cancer diagnostics.
Want to test your assay under real-world conditions? Meridian’s oncology-focused qPCR reagents are available in a convenient sample pack, allowing side-by-side evaluation in your own workflow.
Explore Our Oncology-Ready qPCR Solutions:
References:
1. Amoy Diagnostics. (n.d.). AmoyDx® Pan Lung Cancer PCR Panel. Retrieved June 24, 2025, from Amoy Diagnostics website:
https://amoydiagnostics.com/products/amoydx-pan-lung-cancer-pcr-panel
2. Biofidelity. (n.d.). Aspyre® Lung Reagents. Biofidelity. Retrieved August 8, 2025, from
https://biofidelity.com/products/aspyre-lung-reagents/
3. Labani, S., et al., (2014). CareHPV cervical cancer screening demonstration in a rural population of north India. European Journal of Obstetrics & Gynecology and Reproductive Biology, 176, 75–79.
https://doi.org/10.1016/j.ejogrb.2014.03.006
4. Li, W., et al., (2021). Trends in molecular testing of lung cancer in the mainland People’s Republic of China over the decade 2010 to 2019. JTO Clinical and Research Reports, 2(4), 100163.
https://doi.org/10.1016/j.jtocrr.2021.100163