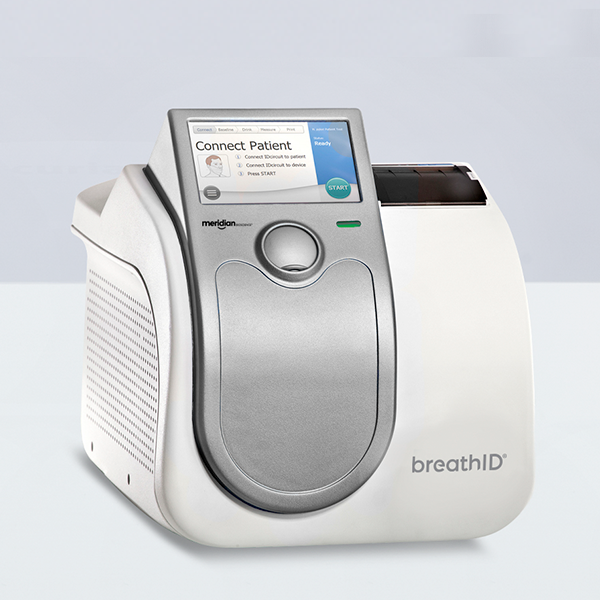
Related Tests
- Gastrointestinal IDkit Hp® One

Kit Components
-
-
Nasal cannula
-
75mg 13C-urea tablet
-
4.3g package of Citrica (citric acid)
-
Drinking straw
-
Package insert
-
Support & Documents
Downloadable PDFs
Webinars & Videos
FAQs
H. pylori urea breath test analysis — 83013
H. pylori urea breath test collection — 83014
Pricing
To order, contact your regional sales representative or use the following contact information:
- Email: sales@meridianbioscience.com
- Phone: +1 800 696-0739
- International Sales Extension: +1 513 271-3000

Get in Touch With Us