In recent years, genetic sequencing has emerged as a powerful tool in healthcare, offering insights into our genetic makeup and predispositions to various diseases. Traditionally, confined to specialized laboratories and research institutions, a genomic revolution is taking place with the democratization of genetic sequencing, where research labs worldwide have improved access to and reduced costs of next-generation sequencing (NGS) technology. Genomic-powered precision health driven by NGS technology is seen as the next major advancement in healthcare. It has the potential to substantially improve patient outcomes by identifying disease-causing variants, enabling the accurate and early detection of cancer, infectious diseases, rare genetic variants, prenatal abnormalities, and pharmacogenetic variations that can impact response to treatment.
Addressing Disparities in Genetic Testing
According to a recent article in Nature Medicine, eighty-six percent of all genomics studies have been conducted in individuals of European descent1, highlighting a stark disparity in the availability and accessibility of genetic testing globally. As a result, the current genomic data available lacks representation from all populations and fails to capture the genetic diversity that exists across different ethnicities and geographical regions. Many diseases exhibit variations in prevalence, severity, and response to treatment based on genetic factors influenced by ethnicity and ancestry. Without adequate representation, the effectiveness of genomic research in addressing the genetic basis of disease in non-European populations is significantly limited.
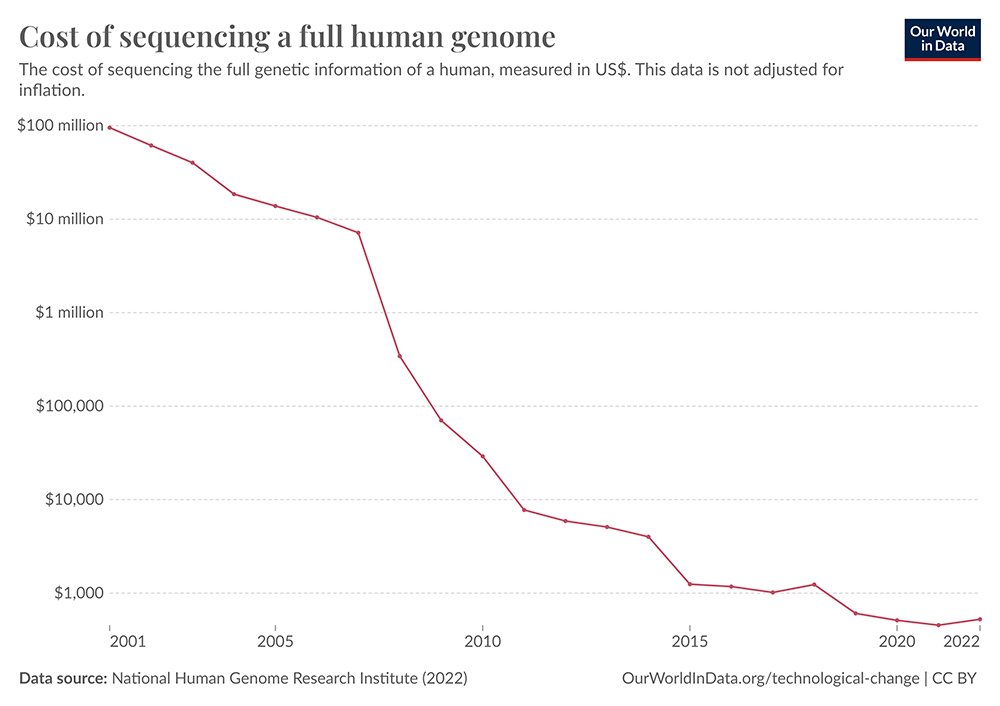
Overcoming Logistical Barriers to Next Generation Sequencing (NGS) Adoption
To achieve widespread adoption of NGS, several logistical barriers must be overcome to enhance its affordability and accessibility. Although the overall affordability has improved since 2007, when sequencing a single human genome cost more than US$10 million2, the cost of sequencing in low-income countries can still be five times higher than in high-income countries because of taxes, and the high cost of analysis, shipment, and infrastructure. Specifically, challenges in low-income countries include coordination of sample handling, reagent cost and procurement, quality-assured procedures, and data management. In addition to logistical complications, from an ethical standpoint, there is also a need to ensure that the benefits of genomic research are equitably distributed and that marginalized communities are not exploited. Efforts to democratize access to NGS technologies and genomic data should ideally be accompanied by initiatives to address disparities in healthcare access, promote inclusivity, and prioritize the needs and perspectives of vulnerable populations3.
Advancements & Technological Growth in NGS
Advances in hardware and software have generally made sequencing faster and more affordable for labs of all sizes, catalyzing the expansion of the NGS market and its application in new environments. Sequencers that accommodate lower sample volumes are accessible at prices equivalent to those for high-volume users, allowing for less sequencing centralization and more democratization and control of samples and the resulting data.
In addition, new sequencing chemistry allows products to be shipped and used without the need for cold chain or temperature-controlled logistics. This innovation addresses a critical challenge faced by many countries, as cold chain logistics can be expensive, logistically complex, and sometimes inaccessible in resource-constrained settings. During the COVID pandemic, due to travel restrictions and disruptions in supply chains, cold chain logistics faced numerous challenges worldwide, highlighting the critical importance of developing alternative, resilient, temperature-stable solutions to support the efficient and reliable distribution of medical products. Enabling laboratories to conduct their own NGS research and analysis without sending samples to other countries overall minimizes costs, leads to faster lead times, and reduces sample handling logistics.
Overall, with the democratization of NGS technology and the continual decline in sequencing costs, its application will continue to expand to new areas, and the incorporation of new tools such as cloud-based NGS informatics platforms, machine learning, and artificial intelligence, will further fuel its role in transforming healthcare globally4.
Steps in an NGS Workflow
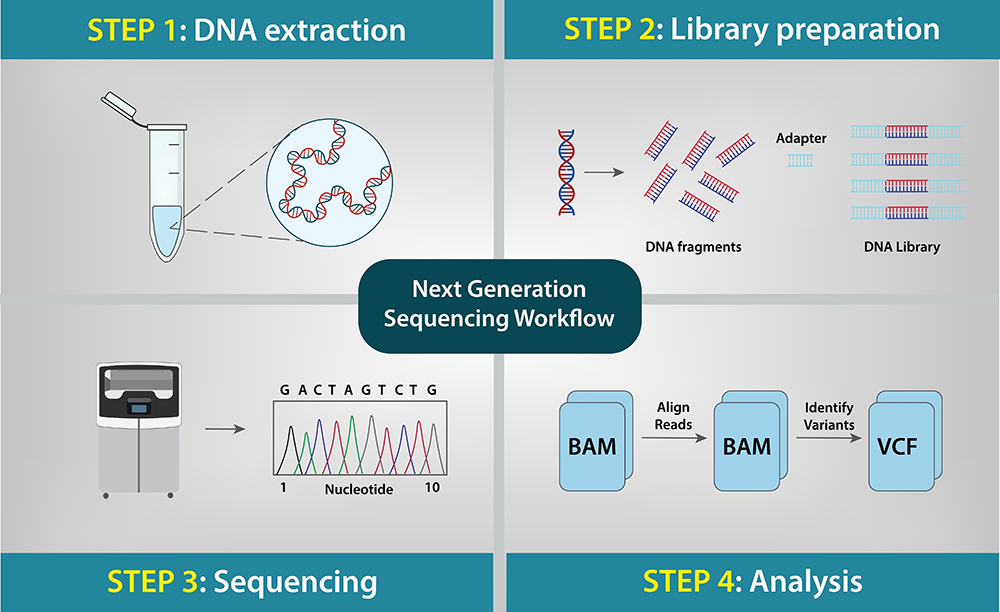
Meridian’s Glycerol-Free Enzymes: Sustainable Solutions for NGS
Meridian has a comprehensive menu of glycerol-free NGS enzymes that are useful in all steps of an NGS workflow. The enzymes are specifically designed for lyophilization and offer a sustainable solution that effectively eliminates the need for cold-chain shipment and storage. They are formulated in a high-concentration format, meeting the demands for miniaturization which is often required for point-of-care devices, and maximizing the sample input quantity to improve assay sensitivity.
To learn more, visit our website:
References:
1. Fatumo, S., et al. A roadmap to increase diversity in genomic studies. Nat Med. 2022 Feb;28(2):243-250. doi: 10.1038/s41591-021-01672-4
2. Stein, J. Expert urges widespread adoption of next-generation sequencing among physicians. Urology Times. 2002 Apr. https://www.urologytimes.com/view/expert-urges-widespread-adoption-of-next-generation-sequencing-among-physicians
3. de Araujo, L., et al. Implementation of targeted next-generation sequencing for the diagnosis of drug-resistant tuberculosis in low-resource settings: a programmatic model, challenges, and initial outcomes. Front. Public Health. 2023 Aug; Sec. Infectious Diseases: Epidemiology and Prevention (11) https://doi.org/10.3389/fpubh.2023.1204064
4. Frost & Sulivan. (2023) Next-generation Sequencing Informatics, Forecast to 2027. https://store.frost.com/global-ngs-informatics-market-forecast-to-2027.html